Chronic obstructive pulmonary disease (COPD)
Chronic obstructive pulmonary disease (COPD) is defined as permanent expiratory airflow limitation due to chronic bronchitis and/or emphysema. Bronchial obstruction is generally progressive and irreversible, but may be accompanied by bronchial hyperreactivity and be partially reversible. Other obstructive causes (cystic fibrosis, obliterative bronchiolitis, etc.) should be ruled out. Smoking is by far the most important etiological factor.
Chronic bronchitis is defined as cough and sputum for > 3 months/year over 2 consecutive years, when other causes of chronic cough have been excluded. Emphysema is defined by a lung characterized by abnormal enlargement of the airspaces above the terminal bronchiole, accompanied by destruction of the alveolar walls without obvious fibrosis.
The prevalence of these syndromes is estimated at 5%, but only 15% have a frank obstructive syndrome that could lead to the diagnosis of COPD. The sex ratio is approximately 2 men to 1 woman, essentially reflecting the male predominance of smoking. With equal risk factors, however, women are considered to be at greater risk of developing COPD than men. Prevalence increases directly with age and poverty (exposure to pollutants and smoking).
Mortality from COPD has risen by 71% over the past 20 years, accounting for 2-5% of all deaths, mainly in developing countries. This figure is probably underestimated (deaths from right heart failure, the ultimate outcome of COPD, are frequently classified as cardiac). It is the 3rd leading cause of death after cardiovascular accidents and cancer. It is the cause of 10% of hospital admissions in pneumology.
NB: simple chronic bronchitis in smokers, with no obstructive syndrome (no exertional dyspnea or abnormalities on examination), has an excellent prognosis when smoking is stopped.
Etiologies, risk factors and aggravating factors
Chronic bronchitis is linked to irritation, leading to hyperplasia of the bronchial wall, scarring and mucous hyperproduction. Emphysema is linked to an imbalance between destructive factors (elastase and other proteases released by neutrophils, oxidants) and protective factors (anti-proteases and anti-oxidants) in the lung parenchyma.
Tobacco
A major etiological factor (and aggravating factor in cases with another etiology). It is estimated that 80-90% of COPD is attributable to smoking (Relative Risk = 10). Active smoking is the main cause, but passive smoking and maternal smoking (alteration of lung development in utero) during pregnancy are also more marginally involved.
The annual decline in mean expiratory volume per second (FEV1) in smokers is on average almost double that of non-smokers. However, only 15% of smokers will develop an obstructive syndrome, and for the same level of smoking, the decline in FEV1 is highly variable between individuals, suggesting genetic susceptibility (role of alpha-1-antitrypsin deficiency? other?) and/or potentiation by other environmental causes.
Cessation of smoking does not restore the FEV1 differential already acquired, but it does restore a downward slope comparable to that of non-smokers, and thus significantly slows the worsening.
Air pollution (SO2, NO2, O3) and domestic pollution
Its role is now well established, both as an aggravating factor (increased decompensation and mortality) and as an etiological factor.
Respiratory infections
Respiratory infections have little or no influence on the long-term evolution of the disease, but are a cause of decompensation, and therefore mortality, in severe COPD. In addition, chronic infections (viral, tuberculosis, pseudomonas aeruginosa, etc.) are sometimes considered to be responsible for the genesis of COPD. Finally, it seems that the onset of pneumonia before the age of 2 is predictive of a decline in FEV1 in adulthood.
Professional risks
Exposure to organic dusts (cotton, wood, flax, hemp, cereals, etc.) or non-organic dusts (metal or rock dusts, coal, cement, granite, plastic combustion, etc.), or to gases (SO2, NO2, etc.), is a major occupational risk.
α-1-antitrypsin deficiency (1% of emphysemas)
Alpha-1 antitrypsin_deficiency (AAT) is a hereditary, autosomal recessive disorder of highly variable expression (many subjects are asymptomatic). Common, under-diagnosed and potentially lethal. It should be systematically investigated in selected patients, even in the presence of another cause (smoking, etc.), since it can be treated with substitutes. As a reminder, of all COPD patients, 1 to 3% are PIZZ and 18% PIMZ.
For the record, other genetic anomalies whose effects are more marginal but cumulative are currently being studied.
Bronchial hyper-reactivity
The existence of this hyper-reactivity and its hypothetical role in the genesis of COPD remain debated, as does whether it is primary or acquired (under the influence of tobacco, for example).
Anatomoclinical forms
These categories are still the subject of controversy, and most patients present a picture combining these forms in a variety of ways.
Chronic bronchitis - large airway lesions - the Blue Bloater
Metaplasia with increased numbers of mucus-forming cells, cartilage atrophy and smooth muscle hyperplasia, leading to increased wall thickness and reduced bronchial lumen. However, there is no clear relationship between the extent of histological lesions and the severity of the obstructive syndrome, nor between cough and sputum and the decline in FEV1.
Patients are typically described as "blue-bloaters": cyanosis, overweight, shortness of breath, moderate dyspnea, high risk of superinfections.
Emphysema - parenchymal lesions - the Pink Puffer
Different types of emphysema can coexist, depending on the location of the lesions in the acinus:
- Centrilobular emphysema: initial lesions in the center of the acinus, within apparently normal parenchyma. Lesions then extend to the rest of the acinus. Predominantly in the upper part of the upper and lower lobes. Found almost exclusively in smokers.
- Panlobular emphysema: enlargement is evenly distributed throughout the acinus. The whole lung is often involved, although the bases are classically the most affected, and lesions tend to coalesce. Encountered in AAT-deficient, elderly non-smokers, but also in smokers.
- Paraseptal emphysema: preferential involvement of distal structures. Lesions are mainly located in the vicinity of fixed structures (pleura, septa, vessels).
- Paracicatricial emphysema: lesions in the vicinity of fibrosing lesions (sequelae of tuberculosis, sarcoidosis, silicosis, etc.), unrelated to the anatomy of the acinus.
- Bullous lesions: can be found in the advanced stages of all types of emphysema. These are areas of airspace dilatation greater than 1cm resulting from a confluence of emphysematous lesions or a "flap" effect on a bronchus.
Patients are typically described as "pink puffers": pink, cachectic, pursed lips, major dyspnea, moderate risk of superinfections.
Small airway lesions
Concept based on the observation that increased resistance (inflammatory infiltrate, reduced caliber, wall thickening) predominates in the peripheral airways, especially in the severe stages of COPD.
Vascular lesions
Found in COPD patients with chronic hypoxemia: accumulation of smooth muscle cells in the intima of small arteries, hypertrophy of the media, etc. It seems that these changes in vascular structure are the main cause of pulmonary hypertension (PH) in these patients, rather than the hypoxic vasoconstriction classically incriminated.
Pathophysiology
Respiratory function in adults deteriorates linearly with age under normal conditions. This degradation, too slow in normal subjects to have clinical repercussions over the course of their lives, is accelerated in COPD patients.
The key factor is the modification of the mechanical properties of the airways and airspaces. Increased airway resistance, reduced dynamic compliance and loss of pulmonary elastic retractility all contribute to pulmonary hyperinflation and expiratory flow limitation. The heterogeneity of these changes results in abnormal ventilation distribution and altered gas exchange function. Two mechanisms can compensate for expiratory flow limitation:
- Extending expiratory time at constant respiratory rate (RR) and tidal volume (VC) → shortening expiratory time and increasing expiratory flow by increasing expiratory muscle contraction.
- Ventilate at rest at high VC since lung flows increase as a function of lung volume. This can lead to deleterious dynamic hyperinflation, which occurs when the duration of expiration is insufficient for lung volume to return to residual volume. Before inspiration → end-expiratory alveolar pressure (PEEP) is positive → inspiratory muscles must develop pressure = PEEP before inspiration becomes effective → increased ventilatory work and reliance on accessory muscles... when the muscles' ability to generate ventilation at rest is already impaired...
In COPD patients, the ventilatory response to variations in hematosis is reduced (secondary to unequal ventilation/perfusion ratios, alveolar hypoventilation, disturbances in O2 diffusion, shunt effect). However, it seems that a decrease in chemoreceptor sensitivity is less to blame than an increase in mechanical inspiratory load limiting the ability to generate an increase in motor pressure. Nevertheless, in COPD, chronic hypoxemia and hypercapnia appear to replace central ventilatory control by pCO2 in normal subjects with control by pO2 → avoid "systematic" oxygen administration during decompensation, and always be cautious about the need for oxygen therapy in COPD (risk of depressing the respiratory center → carbonarcosis).
Clinical
- Anamnesis:
- Look for chronic productive cough (> 3 months/ years over > 2 years) ++ morning, slowly progressive exertional dyspnea (poor prognosis), chest pain (rule out coronary artery disease, gastroesophageal reflux, infection), hemoptysis (rule out bronchial neoplasia).
- Look for exposure to risk factors (++ tobacco).
- Physical examination :
- Mobilization of accessory respiratory muscles, possibly hypertrophied
- Exhalation time > 4 seconds
- Pursing of lips at end of exhalation
- Thorax barrel-shaped, distance between thyroid cartilage and manubrium reduced to less than 4 fingerbreadths
- Nicotine pigmentation of fingers, cyanosis, digital hippocracticism
- Diminished vesicular murmur, snoring bronchial rales, cough, wheezing, hypersonic percussion
- Possible signs of chronic pulmonary heart disease: lower limb edema (LLE), jugular turgor, hepato-jugular reflux (HJR)
- Asterixis in severe hypercapnia (metabolic encephalopathy)
Poor prognostic factors
Age over 65, severe dyspnea, bronchospasm, rapid rate of FEV1 decay, large fixed component of obstructive syndrome, frequent superinfections/decompensations, signs of right heart failure, heterogeneous scintigraphy, nocturnal desaturation, hypercapnia, persistent smoking.
The main causes of death are acute decompensation, pulmonary embolism, pneumothorax, associated bronchial neoplasia, pulmonary heart or associated cardiovascular pathologies.
Additional tests
The examination necessary and sufficient for diagnosis is the RFTs.
- Chest X-ray: sometimes normal, signs of thoracic distension, rail opacities (thickening of the bronchial walls), signs of emphysema (hyperclartes often in the upper lobes, peripheral vascular rarefaction, downward displacement of the scissures, flattening of the domes, etc., sensitivity 60 to 85%).
- Thoracic CT-scanner: confirms the diagnosis (sensitivity 90%, specificity 100%) and specifies the type of emphysema, looking for associated lesions (cancer?) and complications
- Gasometry: hypoxemia, sometimes hypercapnia (sign of severity)
- Biology: secondary polycythemia (late stage)?
- Respiratory function tests (RFTs): obstructive syndrome (decrease in FEV1 < 80%, PEF and Tiffeneau < 70%, increase in RV and FRC) irreversible/ only slightly reversible +- DLCO and compliance
- Electrocardiogram (ECG)
- Cardiac ultrasound: degree of PH, right and left functions.
- Fibroscopy :
- To be performed in the event of radiological abnormality or sudden changes in symptomatology (e.g. hemoptysis).
- Allows bacteriological and histological sampling.
- Perfusion + ventilation scintigraphy: serves as a reference for the evolution of parenchymal functionality and as a comparative basis for a possible future diagnosis of pulmonary embolism.
- Right heart catheterization: to be performed in cases of reduced exercise tolerance without obvious worsening of the obstruction, when cardiac echocardiography is inconclusive, in cases of suspected associated chronic thromboembolic disease, in cases of associated left heart disease to assess LVEF, preoperatively.
- ENT and stomatological work-up in search of infectious foci in the event of repeated exacerbations.
Chronic respiratory failure and chronic pulmonary heart disease
Clinical features: progressive dyspnea with cyanosis, signs of right heart failure. Chest X-ray may show cardiomegaly and large pulmonary arteries. Gazometry: hypoxia-hypercapnia with more or less compensated respiratory acidosis.
Acute decompensation - COPD exacerbations
There are many definitions of a COPD exacerbation, and they are still the subject of much debate. The most frequently used, for its simplicity, is still based on Anthonisen's criteria.
Anthonisen criteria
Based on 3 cardinal symptoms (dyspnea, sputum volume, sputum purulence), they are still the most frequently used to define exacerbation and severity of COPD:
- Severe exacerbation (type 1) = increase in the 3 cardinal symptoms
- Moderate exacerbation (type 2) = increase in 2 cardinal symptoms
- Mild exacerbation (type 3) = increase in 1 cardinal symptom + 1 of the following: fever with no other etiology, lower respiratory tract infection within the last 5 days, increase in inspiratory wheezing, increase in cough, 20% increase in HR or RR.
GOLD criteria
The GOLD criteria are an attempt to clarify the definition. However, they have not been shown to be superior to the Anthonisen criteria, and are more difficult to use in outpatient practice.
- Severe exacerbation = moderate exacerbation + gasometry showing hypercapnia and acidosis
- Moderate exacerbation = mild exacerbation + 3 of the following criteria: dyspnoea VAS > 5, RR > 24/ min, HR > 95 bpm, SaO2 AA (or with usual O2th) < 92% at rest (or > 3% fall from known baseline), CRP > 10 mg/l
- Mild exacerbation = worsening over < 14 days of dyspnea and/or cough and sputum.
Evaluating the indication for hospital care
There is no precise criterion, and the clinician must rely on a combination of severity factors: sudden worsening of rest dyspnea, signs of respiratory distress, confusion, RR, HR, SaO2, comorbidities, poor response to outpatient treatment, lack of adequate support at home, etc.
Causes of decompensation and generalities
Look for a triggering cause (respiratory infections 47%, heart failure 25%, arrhythmia 4%, extra-respiratory infection 3.9%, bronchial cancer 3.3%, surgical complication 1.6%, pulmonary embolism 1.4%, pneumothorax 1%, treatment with β-blockers/ benzodiazepines/ antitussives/ diuretics, chest trauma, therapeutic incompliance, insufficient/ excessive oxygen therapy, atelectasis on mucous plug). However, in over 25% of cases, no triggering factor is identified.
Look for signs of severity + triggers + complications:
- Clinical: right heart failure, neuro-psychiatric deterioration, polypnoea, paradoxical breathing [depressed inspi abdo], mobilization of accessory respiratory muscles and draught, cyanosis, asterixis, hypertension, tachycardia, hemodynamic instability. Signs of pulmonary embolism (PE) or deep vein thrombosis (DVT)?
- Gasometry: hypercapnia, hypoxemia ? PaO2 < 60mmHg resistant to treatment + low systolic blood pressure should rule out pulmonary embolism
- ECG + cardiac ultrasound: arrhythmias? ischemic distress? signs of PH? heart failure?
- Biology: inflammatory syndrome ? organ failure ? pro-BNP and cardiac enzymes ? (d-dimers)
- Peak flow meter: < 100 ml/min = severe decompensation!
- Arguments for an infectious origin: fever (++ bacterial if persists for more than 3 days), increased sputum volume and/or purulence. The presence of ENT signs and the absence of fever suggest a viral origin.
Therapeutic management - Treatments
Background treatment
The historical GOLD classification, both diagnostic and therapeutic, has been replaced by the GOLD - ABE classification:
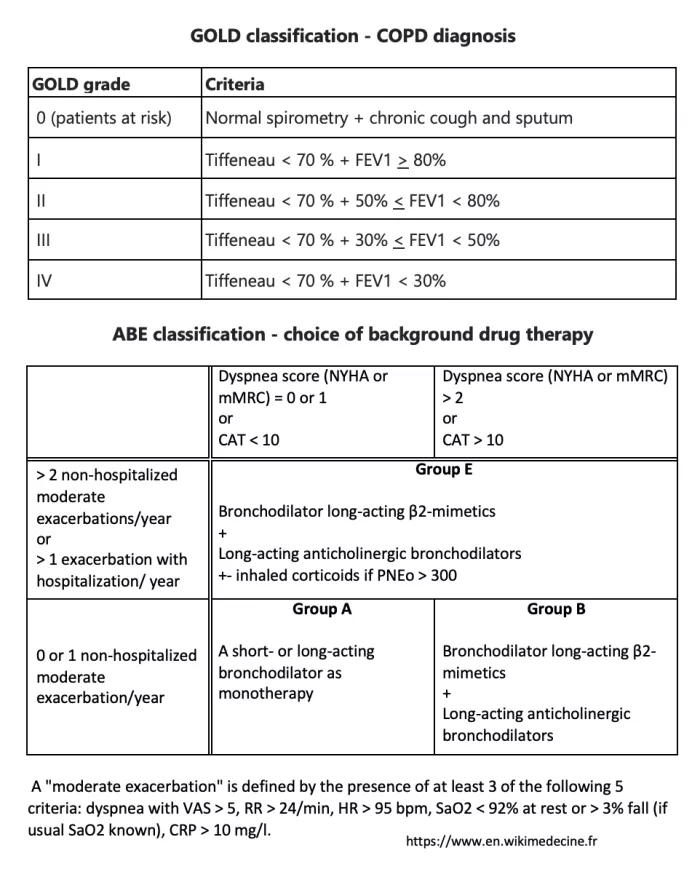
In all cases (including GOLD 0): smoking cessation, search for and removal of other risk factors, physical reconditioning if possible. Flu, pneumococcal, COVID19, whooping cough and VZV vaccinations should be considered.
Treatment is based on bronchodilators: β2-mimetic sprays and short- or long-acting anticholinergic sprays.
Inhaled corticosteroids have a more limited indication. They should be considered from the outset in group E patients with NEP > 300/µL, or as an escalating therapy in group E patients with NEP > 100/µL who continue to have exacerbations. Long-term oral corticosteroid therapy should only be considered in exceptional cases (long-term benefit/risk ratio often unfavorable).
Theophylline per os (xanthine, long-acting, 2x/day 5mg/ kg, check serum levels [target: 5 to 15 µg/ml]) now plays only a marginal role (toxicity, limited positive effects).
The value of roflumilast (NSAID) or macrolides as a last-line treatment is still debated.
Mucolytics and mucoregulators have not been shown to be effective.
The benefits of long-term oxygen therapy in severe COPD patients are no longer in doubt (nasal cannula, 1 to 2 l/ minute, goal: PaO2 > 60 mmHg, > 15 h/d) with PaO2 < 55 mmHg or SaO2 < 88%.
Long-term assisted ventilation (NIV/tracheostomy ventilation) in severely unstable patients should be adopted only as a last resort, as its benefit has not yet been established. Surgical indications (resection of bullae, lung volume reduction for diffuse emphysema, lung transplantation) should be discussed by a multidisciplinary team.
Management of exacerbations
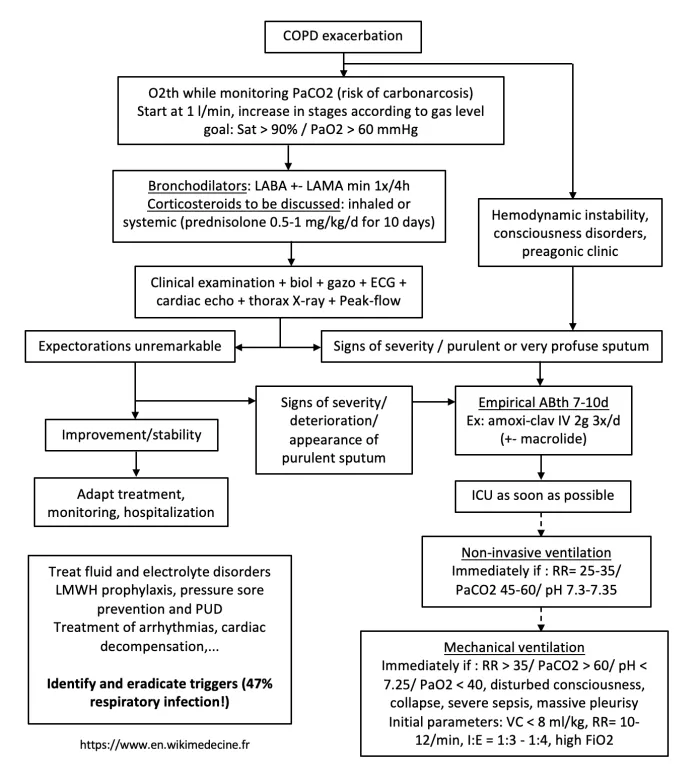
Empirical antibiotic therapy should certainly be initiated in the following situations:
- Acute exacerbation with respiratory distress
- Anthonisen moderate-to-severe acute exacerbation (2 or 3 cardinal symptoms) of moderate-to-very-severe COPD (GOLD 2 to 4).
The indication for antibiotics in other situations should be discussed on the basis of clinical arguments and additional tests. Note that viral exacerbations generally have a longer recovery time than bacterial exacerbations. Examples of empirical regimens:
- Bronchial superinfections requiring antibiotic therapy: augmentin / cefuroxime. Moxifloxacin if IgE-mediated pen allergy or lack of response to 1st-line treatment or suspected enterobacteria. Ciprofloxacin or ceftazidime if Pseudomonas aeruginosa suspected (= previous colonization, hospitalization or recent antibiotic therapy, > 4 courses of antibiotics / year, FEV1 < 30%).
- The presence of clinico-biological arguments for a pulmonary focus requires treatment of pneumonia with co-morbidity, even in the absence of a radiological focus.
Mortality of patients admitted for decompensation is ~10%. Mortality at 6 months varies between 21% and 36% in the event of recurrence within 6 months (which concerns 50% of patients → importance of follow-up to avoid rehospitalization!!)... and in the event of recourse to mechanical ventilation, mortality rises to 43%.
Any exacerbation requiring hospitalization calls for a pneumological assessment around 6 weeks after discharge (resolution? deterioration in respiratory capacity? need to increase background treatment?).
Secondary prevention and prognosis modification
In addition to providing symptomatic background treatment and preventing episodes of superinfection, it is of the utmost importance to convince COPD patients presenting with chronic cough and/or sputum, or with emphysema, to stop exposing themselves to the factors that contribute to their condition (mainly tobacco) as soon as possible. Stopping such exposure will generally produce little clinical improvement (hyper-reactivity component), but is the first step towards improving long-term prognosis. If exposure is stopped, the patient's respiratory function will return to a deterioration curve similar to that of a normal subject. The sooner exposure is stopped, the greater the gain in survival (up to an almost normal morbi-mortality for GOLD 0 patients).
Identifying patients with α-1-antitrypsin deficiency and providing them with special care is also of major importance in halting the progression of the disease.
Bibliography
GOLD, At a glance outpatient management reference for chronic obstructive pulmonary disease (CPOD), 2015
GOLD, Global strategy for the diagnosis, management and prevention of COPD 2023
Agusti A et al., GOLD 2023 : Highlights for primary care, npj Primary Care Respiratory Medicine, 2023
Cooper B et al, Remote Patient Monitoring for the Detection of COPD Exacerbations, Int J Chron Obstruct Pulmon Dis
Sørheim IC et al, Gender differences in COPD: are women more susceptible to smoking effects than men ?, BMJ, 2009