Craniocervical arterial dissection
The incidence of craniocervical artery dissections is around 4/ 100,000 inhabitants/year. The average age of onset is around 40. They account for 20% of ischemic strokes in people under 50. Early diagnosis is crucial, as early treatment effectively reduces the risk of stroke.
Elements of physiopathology
Arterial dissection is a rupture of one or more layers of the arterial wall, usually limited to the intima, allowing blood to enter and cleaving the wall with an intraparietal hematoma. This leads to narrowing, or even occlusion, of the arterial lumen.
Craniocervical dissections can result in local signs (due to compression of neighbouring structures) and signs of cerebral or retinal ischemia, which can occur from a few hours to 1 month later, due to thrombo-embolic phenomena from thrombi formed on the dissected artery, or to a low flow downstream of the stenosis of the arterial lumen.
The most common are extracranial internal carotid and vertebral arteries between the atlas and axis. Dissections of intracranial arteries are thought to be rarer, but their incidence may be underestimated because they are more difficult to diagnose.
Etiologies and contributing factors
- Spontaneous dissections (more than half of all cases)
- Contributing factors: uncontrolled hypertension, Marfan, Ehlers-Danlos, polycystic kidney disease, fibromuscular dysplasia, arterial elongation, etc.
- Traumatic dissections
- High-energy trauma (rolling accidents ++)
- Cervical manipulation
- Iatrogenic dissections in endovascular procedures
- Infectious or inflammatory vasculitis
Clinical picture according to site of dissection
Dissection of an intracranial artery should always be suspected if local signs (cervicalgia, headache, Claude-Bernard-Horner) precede signs of cerebral ischemia, ++ if there is evidence of recent trauma.
Extra-cranial internal carotid artery dissections
Bilateral in 14% of cases.
Local signs (isolated in 25% of cases):
- Headaches (++ frontal and periorbital) and/or neck pain (65 to 75% of cases).
- Claude-Bernard-Horner syndrome due to pericarotid sympathetic compression (30 to 50% of cases)
- Tinnitus (~ 5%)
- Last cranial nerve paralysis (rare, ++ lingual paralysis)
Ischemic signs (occurring in 75% of cases):
- Ischemic strokes, transient (TIA) or permanent, ++ in the territory of the middle cerebral artery (MCA)
- Local signs follow from a few hours to 1 month, but 80% occur within 1 week
- Neuro-ophthalmic symptoms and signs in 63% of cases
- Transient monocular blindness (on retinal emboli, ~30% of cases), ischemic optic neuropathy (~2.5%), oculomotor paralysis (<1%)
Primary carotid artery dissections
These are most often post-traumatic, as part of the extension of an aortic dissection.
Intracranial internal carotid dissections
These usually lead to severe headaches, followed by massive ischemic strokes with a poor prognosis.
Extra-cranial vertebral dissections
Bilateral in 30-60% of cases.
Local signs (in 75% of cases): headache (++ occipital) and/or neck pain.
Ischemic signs (in 75% of cases): ischemic strokes in the vertebro-basilar territory (bulb, trunk, cerebellum, occipital lobes). Wallenberg syndrome (33% of cases) is highly suggestive.
Intracranial vertebral and/or basilar trunk dissections
These are very rare. Frequently the cause of subarachnoid hemorrhage (SAH) +- associated with ischemic stroke of the brain stem.
Multiple dissections
Multiple lesions are not uncommon. Vertebral dissections are bilateral in 30-60% of cases, depending on the series, and associated with carotid dissection in ~30% of cases. The occurrence of multiple dissections should prompt a search for underlying vasculopathy.
Screening in a post-traumatic context
Cervical dissections are not uncommon in trauma, particularly high-energy trauma, and are all too often diagnosed after the onset of an ischemic stroke. Conversely, it is neither feasible nor desirable to perform imaging in all cases.
In such cases, it is currently recommended to perform cervical CT angiography or conventional angiography in the presence of one of the modified Denver criteria:
- Presence of one of the following suggestive signs:
- Cervical murmur in a patient < 50 years of age
- Expansive cervical hematoma
- Arterial hemorrhage
- Neurological abnormality not explained by established lesions
- Appearance of an ischemic patch on a follow-up cerebral CT scan
- Presence of a risk factor = high-energy trauma + one of the following criteria:
- Vertebral subluxation or vertebral fracture involving a transverse foramen or C1-C3 fracture
- Lefort II or III fracture
- Skull base fracture involving the carotid siphon
- Diffuse axonal lesions with Glasgow < 6
- Hanging with anoxic lesions
Urgent investigations
None of these examinations can formally rule out a dissection → are to be used in synergy: cervical (+- transcranial) ultrasound-Doppler + angio-MRI or angio-CT-cervico-cranial scan.
These examinations are urgent because:
Significant risk of (re)occurrence of stroke.
Regression of dissection images is frequent and can be rapid, while the risk of thrombo-embolic events persists.
+ Stroke assessment, if applicable.
Cervical (+- trans-cranial) Doppler ultrasonography
This is the first-line examination, as it is minimally invasive and readily available. Cervical echo-doppler has good sensitivity, except for high dissections.
Transcranial duplex, to assess downstream intracranial hemodynamic effects, is now rarely used.
(Angio)-MRI or angio-CT-scanner
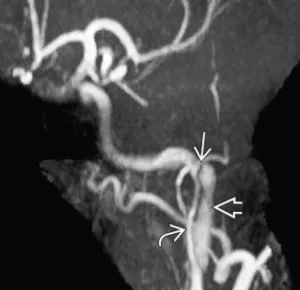
MRI (ask for a proton density sequence) enables visualization of the parietal hematoma, while angio-MRI enables assessment of its extension and impact on the arterial lumen and intracranial flow. The sensitivity and specificity of MRI, which is still recommended by most authors as the 1st-line examination, are poorly determined, and the examination is not easily accessible in emergency → angio-CT-scanner is generally preferred.
Conventional arteriography
Constitutes the Gold Standard (DSA, subtraction arteriography) but is now rarely performed due to its invasive nature and advances in MRI and CT scanning. To be performed in cases of strong clinical suspicion with non-contributory or poorly contributory MRI. It can also be used to detect fibromuscular dysplasia of the renal arteries.
Prognosis - follow-up
The prognosis depends above all on the occurrence and clinical severity of a possible ischemic stroke. Overall, the prognosis for extracranial dissections is favourable (especially for vertebral dissections).
- Internal carotid dissection → 4% death - 25% moderate to severe deficits - 21% minor deficits - 50% no sequelae
- Vertebral dissection → 6% death - 11% moderate to severe deficits - 21% minor deficits - 63% no sequelae
After normalization of the wall, the risk of recurrence is low (1%/year) → in case of recurrence: systematic search for an underlying favoring disease.
Therapeutic management - Treatments
Generally speaking, there is little or no EBM on the management of craniocervical artery dissections, particularly with regard to the choice between antiplatelets and anticoagulants. Management varies from center to center.
There is no recommendation as to the usefulness of long-term radiological follow-up, even in cases of persistent arterial wall anomalies.
General considerations
- Strict decubitus position, feet elevated (hemodynamic stroke prevention): controversial
- Management (blood pressure control, etc.) of a possible ischemic stroke and non-specific measures according to usual considerations
- In particular, there is no EBM justifying not implementing IV and/or IA thrombolysis procedures for eligible patients. We can only recommend particular clinical and radiological attention to possible bleeding complications.
- In the event of secondary or iatrogenic intracranial haemorrhage, specific management before considering remote anti-thrombotic treatment on the basis of neurosurgical advice.
- Screening and management of cardiovascular risk factors
Extra-cranial dissections
- Depending on the center's preferences and assessment of bleeding risk: choose between antiplatelet therapy and anticoagulation (although the latter is often preferred, there is no demonstrated difference in terms of benefits and risks).
- Ex in case of antiplatelet choice: acetylsalicylic acid (ASA) 100 to 300 mg/day → MRA check at 6 months → ASA maintained if arterial wall anomalies persist (stenosis, parietal irregularities, secondary aneurysms,...)
- Heparin at therapeutic dose → early relay with vitamin K antagonists (VKAs) or new oral anticoagulants (NOACs) to be continued :
- 6 weeks if dissection without associated stroke → relay with acetylsalicylic acid (ASA) 100 to 300 mg/day
- 6 months if associated ischemic stroke → MRA (or arteriography) check-up
- If normalization → relay with ASA 100 to 300 mg/day for 1 year
- If stenosis, parietal irregularities or aneurysms → continue AVK for 3 months until further imaging
- Surgical or endovascular indications are exceptional (rain of transient ischemic attacks, formation of a sub-occlusive stenosis with poor recovery of the polygon of Willis,...) and not codified
Intracranial dissections
- Theoretical risk of secondary subarachnoid hemorrhage (absence of external elastic boundary and thinning of other tunics of intracranial vessels) → theoretical contraindication to anticoagulants → current consensus: symptomatic treatment → antiplatelet treatment (e.g. ASA 160 mg/ d) and MRA control at 6 months
- If arterial stenosis persists: maintain antiplatelet therapy
- Endovascular indications are exceptional and not codified.
Bibliography
Arthurs ZM et al., Blunt carotid and vertebral artery injuries, Injury, 2008, 39:1232–41
Biller J et al., Cervical Arterial Dissections and Association With Cervical Manipulative Therapy. A Statement for Healthcare Professionals From the American Heart Association/American Stroke Association, Stroke, 2014; 45: 3155-3174
Biffl WL et al., Optimizing screening for blunt cerebrovascular injuries, Am J Surg, 1999, 178(6):517-22
Bromberg WJ et al., Blunt cerebrovascular injury practice management guidelines: the Eastern Association for the Surgery of Trauma, J Trauma, 2010, 68(2):471-7
Cothren CC et al., Cervical spine fracture patterns mandating screening to rule out blunt cerebrovascular injury, Surgery, 2007, 141(1):76-82
Engelter ST et al., Antiplatelets Versus Anticoagulation in Cervical Artery Dissection, Stroke, 2014; 45: 3155-3174
Liebeskind DS et Saver J, Spontaneous cerebral and cervical artery dissection: Treatment and prognosis, UpToDate, Sept 2015
Miller PR et al., Prospective screening for blunt cerebrovascular injuries: Analysis of diagnostic modalities and outcomes, Ann Surg, 2002, 236:386–93
Osborn AG et al, Brain, Elsevier, 2018

